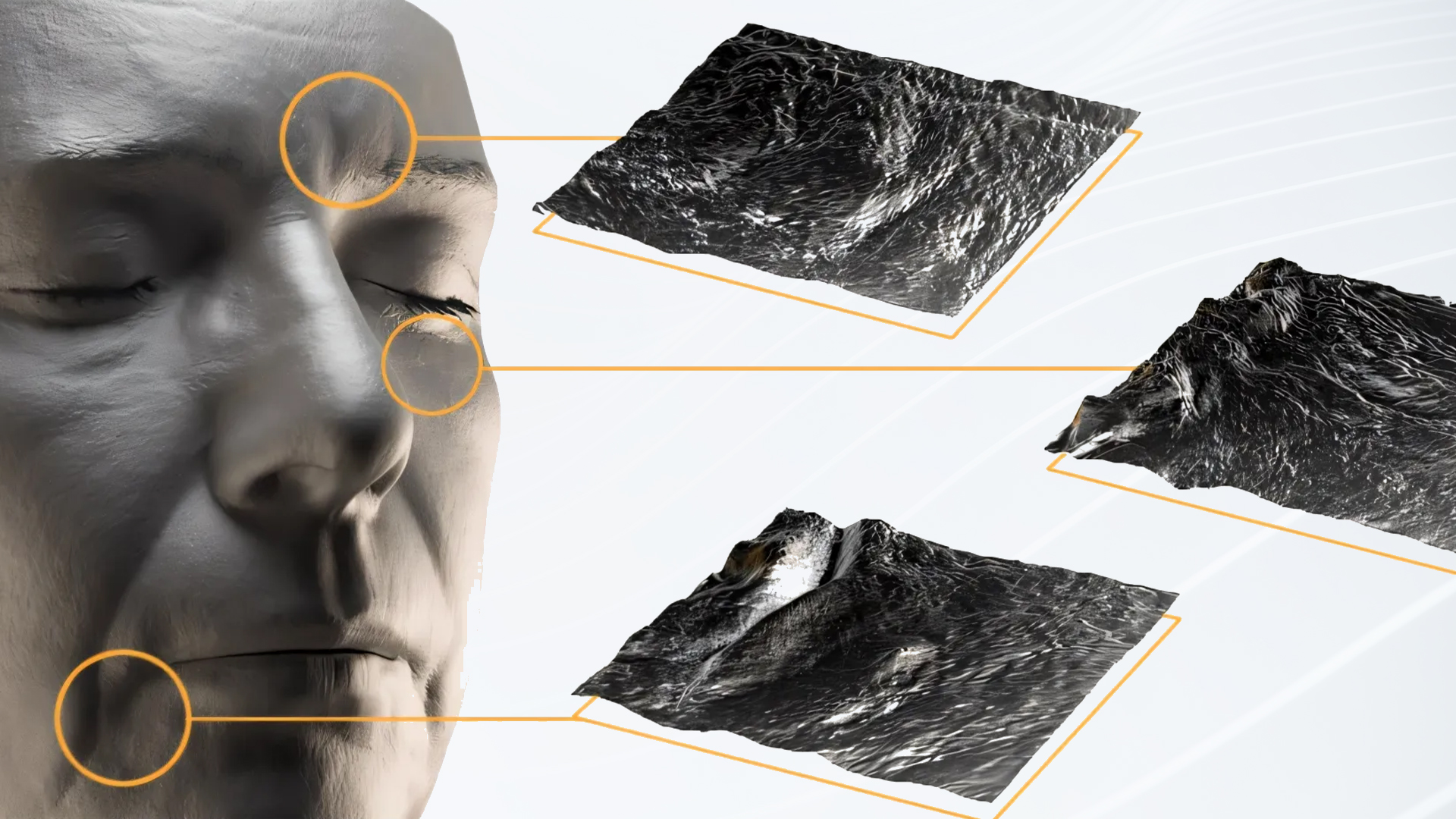
In skincare product development, efficacy testing is a critical part of clinical trials. Traditional methods often involve multiple tools and procedures, making the process time-consuming, fragmented, and prone to biases. Cydolia’s 3D clinical trial technology provides a precise, comprehensive, and efficient solution for efficacy testing, streamlining data collection and offering scientifically validated insights.
Challenges in traditional efficacy testing
Traditional efficacy testing methods for skincare products require various instruments to measure different skin parameters such as elasticity, hydration, and wrinkles. These methods present several challenges:
- Extended timelines: The need for repeated calibration and repositioning of tools increases the duration of trials.
- Fragmented data: Each tool captures localized data, limiting the ability to view the overall impact of a product on the skin.
- Potential biases: Variations in lighting, facial expressions, and positioning can introduce inconsistencies across different trials.
How Cydolia’s 3D technology solves these problems in efficacy testing
Cydolia’s 3D technology transforms efficacy testing by capturing optical, metric, and mechanical skin properties in a single scan, eliminating the need for multiple tools. Our system provides a comprehensive view of product efficacy, ensuring more consistent, reproducible results, all without the need for repositioning or recalibration.
Precision
Cydolia’s system offers holistic capture of the full face, providing the most detailed insights into skin properties. This comprehensive capture allows for post-scan analysis, including the ability to define regions of interest (ROI) on the 3D model after reconstruction, enabling focused analysis on specific areas as needed. By extracting a wide range of skin properties, the system can measure standard skin parameters and create new custom parameters for innovative claims.
Speed
With Cydolia’s 3D technology, facial data is captured in seconds, significantly reducing trial timelines. There’s no need for repositioning benches or recalibrating instruments, streamlining the entire process.
Bias-free comparisons
Using state-matching algorithms, Cydolia eliminates biases caused by lighting, facial expression, or positioning. This ensures that results are consistent and reproducible across different subjects and time points, making the data more reliable.
Customization
Cydolia’s technology allows researchers to define regions of interest (ROI) and customize evaluation descriptors. This flexibility ensures that trials can focus on the most relevant parameters while also supporting the development of innovative claims.
Long-term value
The data captured during efficacy testing can be repurposed for marketing, skin atlas, regulatory submissions, and future product developments. The 3D models and texture maps generated during the trial can be reused for before-and-after visualizations, enhancing the value of the trial data.
Portable and scalable
Cydolia’s system is portable and can be deployed on-site, without the need for specialized environments. It can also be integrated into existing CRO protocols, offering flexibility in trial design and execution.
The benefits of using Cydolia’s 3D technology in efficacy testing
Cydolia’s 3D technology addresses many of the limitations of traditional efficacy testing methods. Here are the key benefits:
- Precision: Holistic capture of the full face for the most detailed insights, allowing for post-scan definition of regions of interest on the 3D model.
- Speed: Data collection is completed in seconds, significantly reducing trial timelines.
- Bias-free comparisons: Consistent, reproducible results across different subjects and time points.
- Customization: Tailored evaluation criteria and custom parameters to suit your product’s unique properties.
- Long-term value: Data that can be reused for marketing, regulatory, and future trial use.
- Portable and scalable: The system integrates seamlessly into existing CRO protocols or can be deployed on-site, offering maximum flexibility.